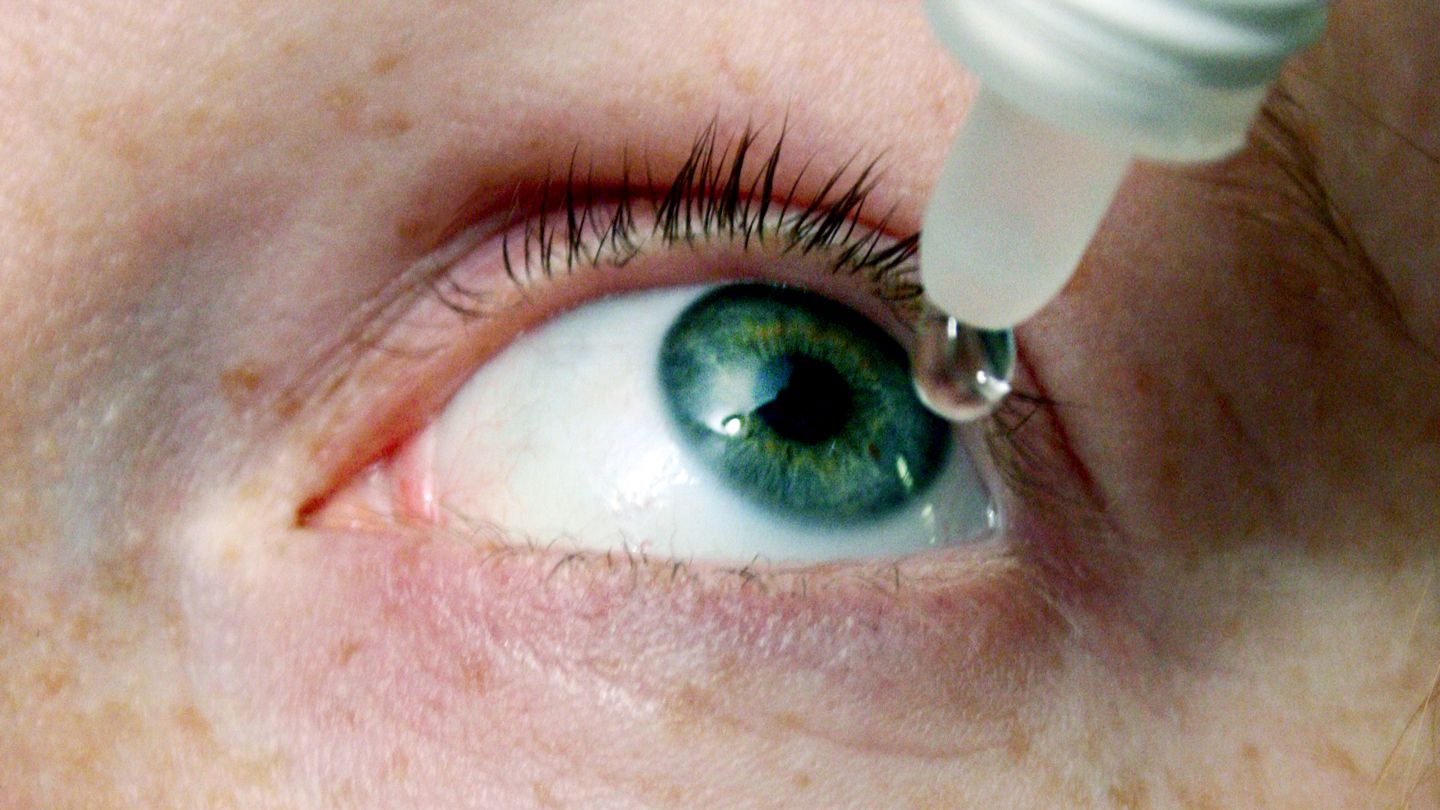
The Centers for Disease Control and Prevention (CDC) is warning consumers to stop using using EzriCare Artificial Tears in the wake of a “multistate cluster” of infections linked to the eye drops.
At least one person has died from a bacterial infection that may be associated with the preservative-free eye drops, the CDC said in a January 20 statement (PDF). “Patient outcomes include permanent vision loss resulting from ocular infection, hospitalization, and death of one patient with bloodstream infection,” the CDC said.
Altogether, CDC investigators have located at least 50 people in 11 states infected with Pseudomonas aeruginosa (PDF), a type of bacteria that is resistant to treatment with antibiotics. So far, the CDC has identified cases in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, and Washington State.
New Mexico public health officials said in a January 10 statement (PDF) that 11 patients who tested positive for P. aeruginosa each had underlying eye diseases and used medicated eye drops or artificial tears prior to their infection. So far, five of these patients have developed vision loss in an eye treated with drops.
The multistate investigation is ongoing, and regulators haven’t determined whether eye drops directly caused these infections, the CDC said. However, the CDC disclosed that most of the cases around the country involved people who developed infections after using artificial tears — most often products from EzriCare.
Bottles of EzriCare Artificial Tears Should Be Discarded
“CDC recommends that clinicians and patients immediately discontinue the use of EzriCare Artificial Tears until the epidemiological investigation and laboratory analyses are complete,” the CDC said.
EzriCare announced in a January 24 statement that the company had not received any consumer complaints, reports of adverse events, or communication from regulators about the ongoing investigation into infections linked to its eye drops.
The company also said it doesn’t manufacture the product under investigation. The EzriCare Artificial Tears were formulated, designed, and imported by Aru Pharma in the United States and were manufactured by Global Pharma Healthcare PVT in India, EzriCare said.
“Nevertheless, and in an abundance of caution, EzriCare recommends that during this evolving situation you discontinue use of any portions of EzriCare Artificial Tears Lubricant Eye Drops you may have until we can discover more details about any potential safety concerns,” EzriCare said. Consumers can contact the company at@ezricare-info.com with any questions or concerns.
What Is the Bacteria Behind These Eye Infections?
P. aeruginosa can cause many types of infections, including pneumonia, bloodstream infections, urinary tract infections, and surgical site infections, according to the CDC. It is one of a growing number of organisms that are resistant to treatment with many commonly prescribed antibiotics.
There have been some previous reports of eye infections from P. aeruginosa, including some cases related to mascara applicators and improper use or care of contact lenses, according to the CDC.
Over-the-counter eye drops may be used to relieve a variety of conditions including dryness, itchiness, and redness. Artificial tears are lubricating eye drops often used to relieve dry eye, according to Mayo Clinic.